The pharmaceutical industry, historically characterized by rigorous regulations and lengthy development cycles, is undeniably undergoing a digital revolution. Digital transformation promises to reshape every facet of how pharma operates, from accelerating drug discovery to optimizing supply chains and enhancing patient engagement. However, managers leading this complex shift often find the path forward nebulous. How can they move beyond abstract concepts and pinpoint concrete avenues for digital value? The answer lies in the power of use cases.
In this article, we will explore the pharmaceutical industry landscape, the challenges pharma companies face, and how Salesforce can address these challenges through various use cases.
The Pharmaceutical Industry Explained
The pharmaceutical industry is primarily focused on the development and distribution of drugs and therapies. It is characterized by a diverse range of stakeholders, including:
- pharmaceutical companies,
- healthcare professionals (HCPs),
- regulatory bodies,
- and patients.
The industry is heavily regulated, with strict compliance requirements to ensure the safety and efficacy of medications.
So, what are the key players in the pharmaceutical industry?
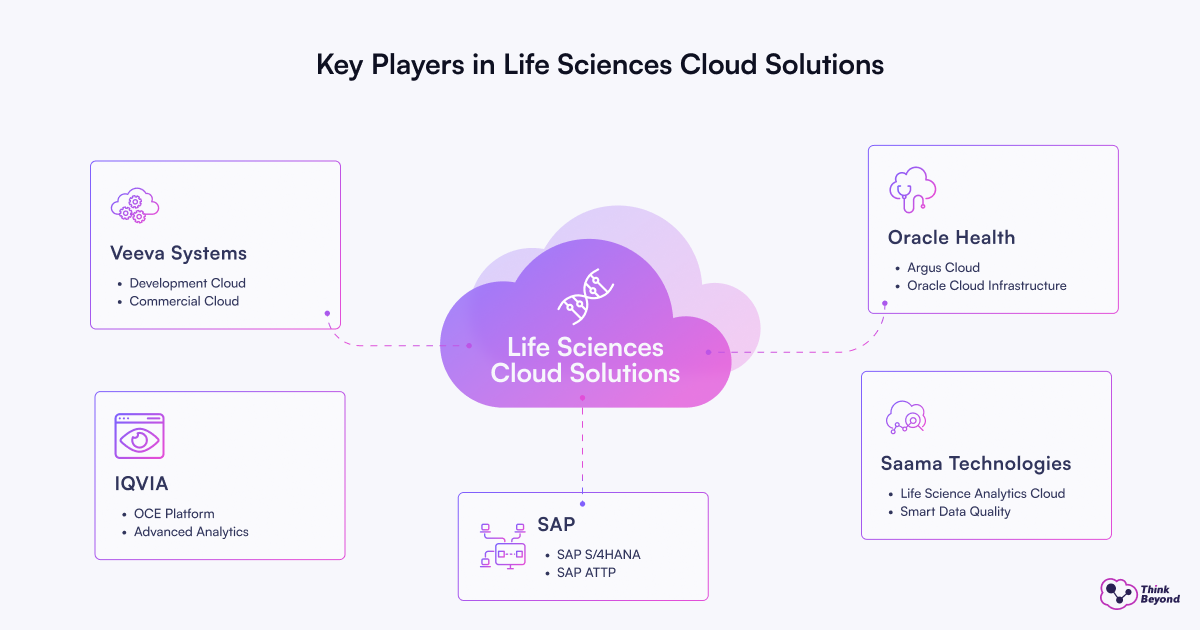
Veeva Systems
Veeva Systems is a leading provider of cloud-based solutions tailored for the life sciences industry. Its platforms—Development Cloud and Commercial Cloud—unify R&D, clinical, regulatory, quality, safety, and commercial operations. Veeva’s Development Cloud streamlines drug development by integrating clinical trial management, regulatory submissions, quality control, and pharmacovigilance into a single platform, eliminating silos and increasing efficiency. The Commercial Cloud connects sales, marketing, and medical teams, leveraging AI and data analytics for customer-centric engagement and faster insights.
After more than a decade of partnership, Veeva announced it would not renew its agreement with Salesforce when it expires in September 2025. There is a five-year wind-down period (2025–2030) during which existing customers can continue using Veeva CRM on Salesforce while they transition to Veeva’s new platform.
Oracle Health
Oracle Health delivers a comprehensive suite of cloud-based solutions for pharmaceutical research, clinical trials, safety, analytics, and EHR integration. Its offerings include Argus Cloud for drug safety, Oracle Cloud Infrastructure (OCI) for scalable computing and data management, and Health Foundation for unified healthcare data and analytics. Oracle’s platforms enable end-to-end management of the drug lifecycle, from R&D and clinical operations to regulatory compliance and real-world evidence generation, supporting integration with EHRs and advanced AI-driven analytics.
SAP
SAP is the backbone of enterprise operations in pharma, offering robust ERP, supply chain, quality, and compliance solutions. Its flagship platform, SAP S/4HANA, integrates core business processes—finance, manufacturing, inventory, and quality management—ensuring real-time visibility and regulatory compliance. SAP Advanced Track and Trace for Pharmaceuticals (ATTP) enables serialization and traceability for drug safety, while the Business Technology Platform (BTP) supports innovation, integration, and analytics. SAP’s solutions are widely used to manage complex supply chains, ensure batch quality, and comply with global regulations.
IQVIA
IQVIA is a global leader in healthcare data, analytics, and AI, providing deep clinical and commercial insights through its OCE platform. The company integrates vast healthcare datasets, advanced analytics, and natural language processing (NLP) to support clinical trial optimization, market access, and real-world evidence generation. IQVIA’s solutions go beyond traditional CRM by embedding real-time intelligence and actionable insights directly into user workflows. The OCE suite includes specialized modules such as OCE Personal, OCE Digital, OCE Optimizer, OCE Connect, and OCE+, catering to various roles from sales representatives to medical science liaisons and brand managers
In 2024, Salesforce and IQVIA announced an expanded global partnership focused on accelerating the development of Salesforce’s Life Sciences Cloud, a next-generation customer engagement platform for the life sciences sector.
Saama Technologies
Saama Technologiespecialiseses in AI-powered clinical analytics and trial data management. Its Life Science Analytics Cloud (LSAC) and Smart Data Quality (SDQ) platforms automate and optimize clinical development processes, reducing time to insights and improving data quality. Saama’s solutions integrate, curate, and analyze clinical trial data, enabling sponsors and CROs to accelerate study timelines, enhance regulatory submissions, and bring therapies to market faster. The company is recognized for its deep expertise in AI/ML for life sciences, with leading pharma companies leveraging its platforms for advanced clinical data management.
Where Pharma Struggles: Key Challenges Explored
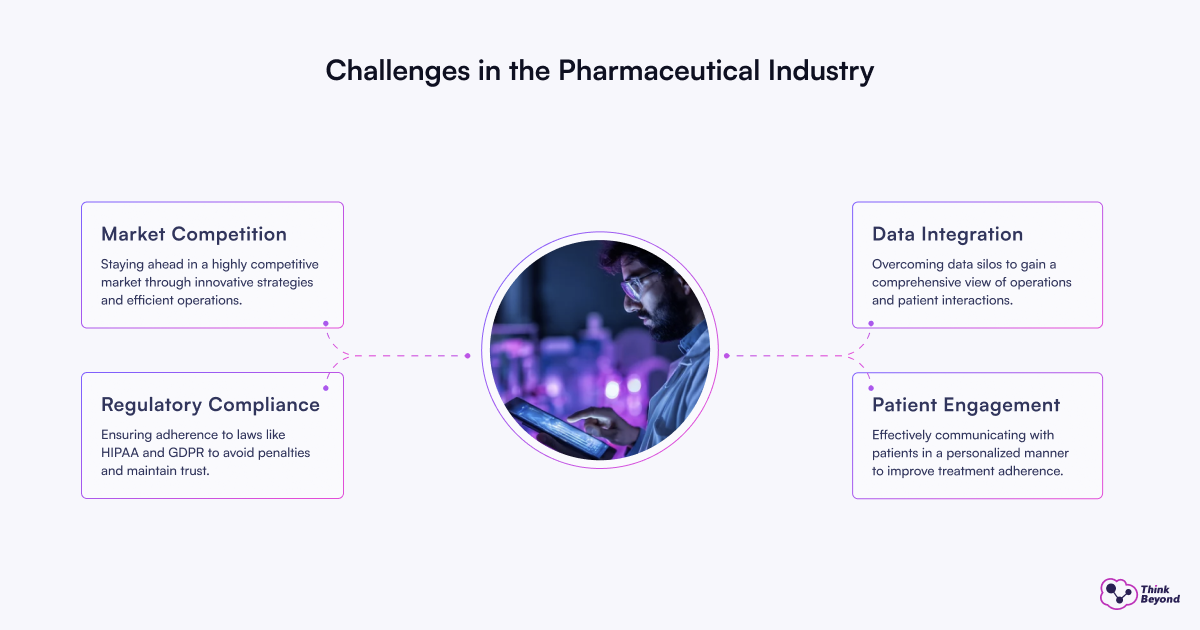
The pharmaceutical industry faces several challenges that can hinder its ability to deliver effective healthcare solutions. These challenges include:
- Navigating the complex landscape of regulations can be daunting for pharmaceutical companies. Ensuring compliance with laws such as HIPAA and GDPR is essential to avoid penalties and maintain trust.
- Many organizations struggle with siloed data across various departments, making it difficult to gain a comprehensive view of operations and patient interactions.
- Engaging patients effectively is crucial for adherence to treatment plans. However, many companies lack the tools to communicate with patients in a personalized manner.
- The pharmaceutical industry is highly competitive, with numerous companies vying for market share. Staying ahead requires innovative strategies and efficient operations.
Salesforce’s Life Sciences Cloud, Health Cloud, Marketing Cloud, and Data Cloud empower pharma teams to connect data, automate processes, and deliver personalized experiences across every stage of the product lifecycle. As Ranjit Gill, IT Director at McKesson, notes, “With Salesforce, we can maximize the digital potential and provide a more flexible and frictionless customer experience”. This commitment to industry-specific innovation is further reflected in Salesforce’s rapid expansion within the pharmaceutical market. According to Salesforce, more than 40 major pharmaceutical customers—including a top three global pharma leader—have signed on to adopt its next-generation life sciences solutions, many of them transitioning from established competitors.
Salesforce in Action: Key Use Cases for Pharma
As of early 2025, more than 1,700 companies around the world are using Salesforce Health Cloud, and a growing number of these are pharmaceutical manufacturers. This shows how quickly Salesforce is becoming a key technology partner in the pharma industry.
So, what makes Salesforce so successful in this space? Let’s take a closer look at how Salesforce solutions are used in the pharmaceutical industry.
Gaining a Complete Understanding of Healthcare Professionals
A pharmaceutical company launching a new specialty drug targeting oncologists can leverage Salesforce’s 360° HCP Relationship View by integrating CRM data, prescription analytics, and conference attendance records. This integration creates unified profiles for healthcare professionals, detailing their prescription history, engagement with clinical content, and preferences for meetings. Sales teams can use these insights, enhanced by AI-driven recommendations, to tailor their interactions, resulting in increased engagement with target HCPs and faster market penetration.
Engaging Healthcare Professionals Across Multiple Channels
For multichannel product education campaigns, Salesforce’s omnichannel HCP engagement capabilities allow pharmaceutical companies to reach healthcare professionals through email, messaging apps, and virtual events. By configuring Marketing Cloud to deliver clinical study summaries via email, enable quick Q&A sessions through messaging platforms, and host live expert sessions online, companies ensure consistent and personalized communication. This approach leads to higher engagement rates and a unified brand message across all touchpoints.
Empowering Patients with Self-Service Portals
When supporting patients with chronic disease medication adherence, companies can use Salesforce Experience Cloud to create patient portals. These portals provide automated medication refill reminders, symptom tracking tools, and treatment journals connected to EHR systems. Patients can also schedule telehealth appointments directly through the portal, resulting in improved adherence, reduced support calls, and better overall health outcomes.
Streamlining Patient Support and Inquiries Through Intelligent Call Centers
In the context of post-vaccine safety monitoring, Salesforce’s call center solutions, such as Salesforce Service Cloud Voice, streamline patient inquiries by routing calls based on symptom severity, language preference, and regional regulations. Each call is automatically logged and categorized, ensuring that urgent cases are escalated quickly and patient concerns are addressed efficiently.
Collecting and Analyzing Patient Feedback to Improve Products and Services
For postmarket drug experience monitoring, Salesforce enables pharmaceutical companies to automate patient surveys at set intervals after prescriptions are filled. Responses, including free-text feedback, are analyzed using AI-powered natural language processing to identify trends and previously unreported side effects, providing valuable insights for product safety and improvement.
Simplifying Consent Management for Global Clinical Trials
Managing patient consent for global clinical trials is simplified with Salesforce’s consent management tools. Companies can deploy dynamic, region-specific consent forms, track disclosures, and maintain detailed audit trails, ensuring compliance with GDPR, HIPAA, and other regulations across multiple countries.
Protecting Patient Data and Ensuring Compliance with Global Privacy Regulations
To address global data privacy requirements, Salesforce offers robust encryption and access control features. By implementing these tools, pharmaceutical companies can protect sensitive data, such as adverse event reports and HCP payment information, while ensuring compliance with diverse international regulations and building patient trust.
Optimizing Pharmaceutical Supply Chains and Inventory Management
Integrating Salesforce with ERP and inventory management systems, such as SAP, allows pharmaceutical companies to optimize vaccine cold chain management. Real-time temperature monitoring, automated reordering, and batch recall simulations help reduce product spoilage and improve supply chain efficiency, especially during large-scale distribution efforts.
Automating Compliance and Regulatory Reporting for Healthcare Professional Programs
For compliance with regulatory requirements in HCP speaker programs, Salesforce can automate validation processes, including fair market value calculations, spend tracking, and content approval workflows. Integration with life sciences platforms ensures that all marketing materials and interactions are properly documented and reported, streamlining compliance and reducing regulatory risk.
Launching Targeted Marketing Campaigns for Rare Disease Treatments
When launching a rare disease drug, pharmaceutical companies can use Salesforce’s marketing automation tools to create targeted campaigns triggered by new diagnosis codes in EHR systems. These campaigns guide healthcare professionals through educational content and patient materials, with analytics tracking the impact on prescription rates and campaign effectiveness.
Integrating EHR Data for Enhanced Real-World Evidence and Patient Insights
Integrating Salesforce with Electronic Health Record (EHR) systems enhances real-world evidence generation by enabling anonymized analysis of treatment patterns and patient outcomes. This integration supports collaboration between healthcare providers and pharmaceutical representatives, facilitates site-specific enrollment forecasting, and accelerates patient recruitment for clinical studies.
Managing Clinical Trial Data Efficiently in Decentralized Research Settings
For decentralized clinical trial monitoring, Salesforce’s clinical data management tools enable integration with electronic patient-reported outcomes devices, centralized dashboards for monitoring trial progress, and automated resolution of data queries. These capabilities streamline trial processes, improve data accuracy, and support timely regulatory submissions.
In all these scenarios, successful implementation relies on phased rollouts, close collaboration with medical and commercial teams, alignment with regulatory requirements, and seamless integration with existing enterprise systems. By applying these Salesforce solutions, pharmaceutical companies can address industry challenges, improve compliance, enhance patient and provider engagement, and achieve greater commercial effectiveness.
Bridging the Gap with Strategic Use Cases and Salesforce Solutions
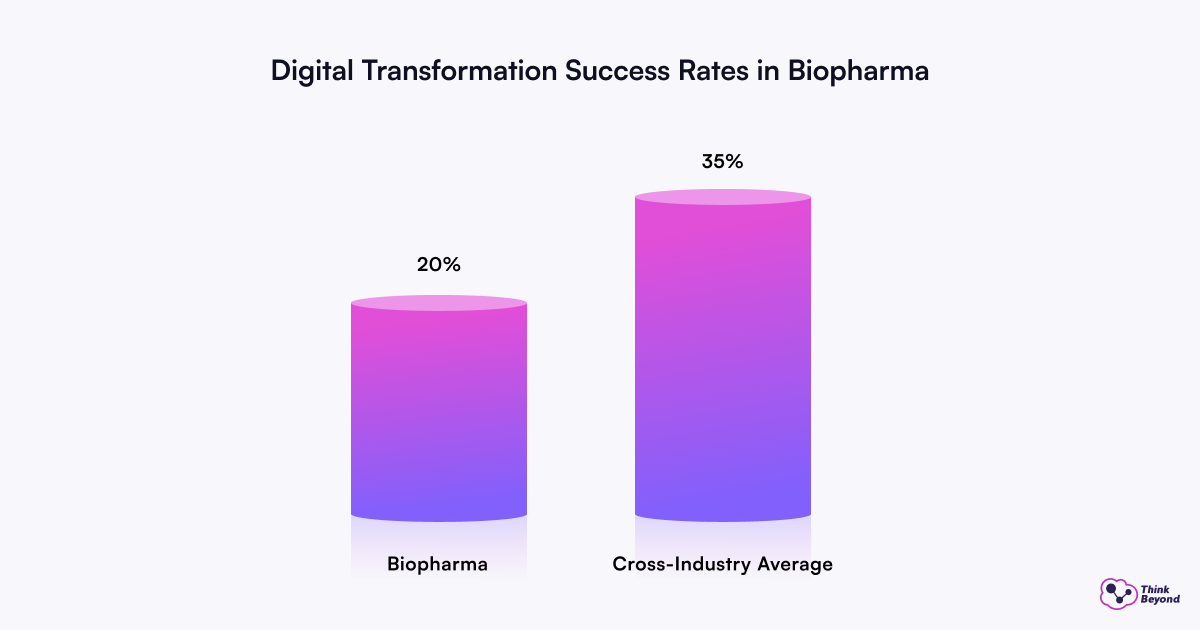
These practical applications of technology are key to success because they provide focus, clarity, and a concrete path forward for change. According to a 2022 report by the Boston Consulting Group (BCG) and a 2025 analysis by Binariks, less than 20% of biopharma companies have successfully executed a digital transformation, compared to a 35% cross-industry average. Nevertheless, the potential benefits are too significant to ignore. For example, a PwC Strategy& report suggests that AI-driven improvements in efficiency and revenue generation could generate an additional $254 billion in annual operating profits for innovative pharmaceutical companies worldwide by 2030. This could potentially increase operating margins from around 20% today to over 40%. Clearly, the “why” for digital transformation is compelling, and use cases provide the “how.”
Salesforce’s emphasis on customer relationship management (CRM), coupled with its specialized cloud solutions and artificial intelligence (AI) capabilities, enables pharmaceutical companies to navigate intricate processes, foster stronger stakeholder relationships, and spearhead digital transformation. These efforts lead to improved patient outcomes, accelerated drug development, optimized commercial strategies, and a stronger competitive edge in the modern healthcare landscape.

